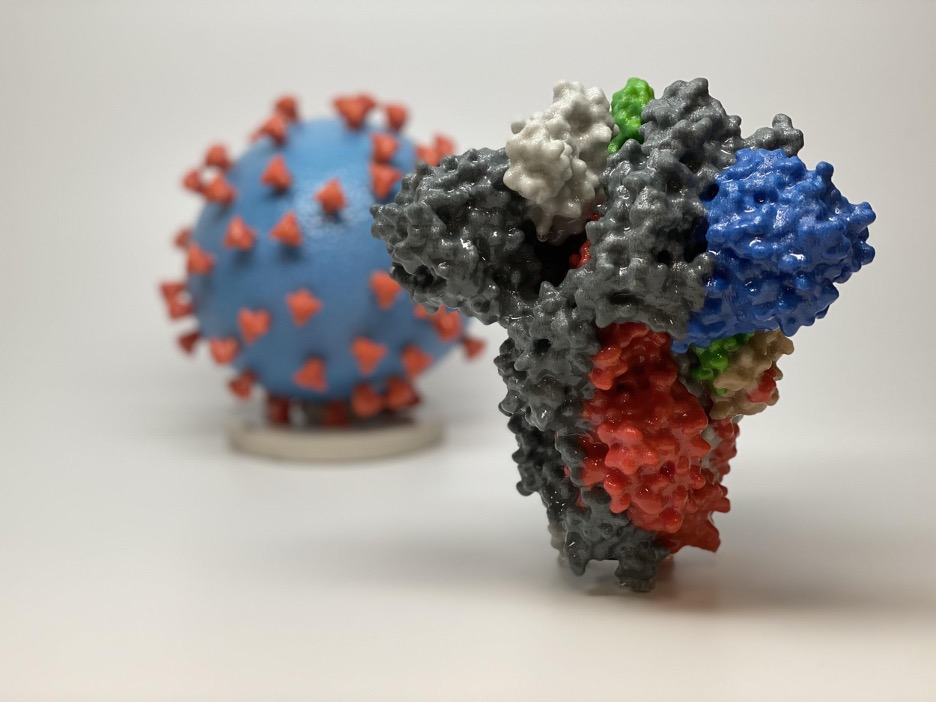
Figure 1: 3D printed models of the SARS-CoV-2 virion (the blue sphere in the background) decorated with spike proteins and a larger model of the spike protein (the multi-colored shape in the foreground; the Receptor Binding Domain is the green section of the model)
Source: Wikimedia Commons
The so-called “UK”, “Brazil”, and “South Africa” variants of the SARS-CoV-2 virus have many commonalities, including that they seem to be more contagious than the previous versions of the virus. Additionally, each variant contains a common mutation: N501Y. This is a change in their 501st amino acid from asparagine (an amide-containing amino acid) to tyrosine (a phenol-containing amino acid). The fact that this mutation has arisen at least three times independently suggests that it likely confers some form of evolutionary advantage. This mutation affects the Receptor Binding Domain (RBD), of the spike glycoprotein that decorates the outside of the free virions (virus particles). A change in this domain may prevent binding to host antibodies and make the variants more infective by altering their interactions with potential host cells (Zimmer, 2021).
As many vaccines (including those made by Moderna and Pfizer that have been approved for use in the US) are designed to make one’s immune system target this spike protein, there is growing concern that an immune response generated through vaccination may not be protective against these variants. Xie et al. demonstrated that antibodies generated by individuals who have received both doses of Pfizer’s vaccine had equal neutralization abilities against the 501N virions or engineered 501Y virions. This is corroborated by Wang and colleagues who showed that antibodies against the virus (produced by natural infection or by inoculation with the mRNA vaccines) were specific for the RBD of the spike protein. However, they also showed that there is a slight decrease in neutralization capacities of antibodies produced by the vaccines against these N501Y mutations. Together, these two studies thus indicate that some of the other mutations unique to the different variants, especially those within the RBD, may be contributing to the slight decrease in antibody neutralization capacity seen.
The RBD of SARS-CoV-2 binds to the ACE2 enzyme on host cells when the spike protein of the virus is in its “open” state, allowing the virus entry into these cells. Teruel et al. demonstrated that the N501Y mutation caused an increase in the likelihood of the spike protein being in the “open” conformation and thus argue that this may be contributing to the increased infection rates being reported among these variants. However, it is still unclear if these changes lead to more severe infections and/or higher mortality rates.
Taking the reduced antibody binding capacity with the increased infection rate, it is quite clear that those worried about the variants might be justified. However, “booster shots” catered towards these variants are being developed (such as the one being developed by Moderna, as reported by Jones). Additionally, Wang et al. discussed that the plasma from recovered and immunized individuals contained antibodies with specificity for the RBD at locations unaffected by the mutations. Thus, B-cells that no longer possess specificity in their antibodies for the mutated virions may become less prevalent in the body as a result of no longer receiving the signals that maintain them, while those that still can bind to the virions despite the mutations will proliferate (LeBien, 2008). So, the body will phase out its ineffective antibodies and rearm itself with weapons perfect for targeting the mutant virus.
As the virus infects more and more people, it will likely continue to evolve and give rise to more variations of its RBD. However, it seems that our antibody-producing B-cells and the scientists working on vaccines are already preparing for these changes.
References
Jones, A. (2021, January 25). Moderna Developing Booster Shot for New Virus Variant B.1.351. The Scientist Magazine®. https://www.the-scientist.com/news-opinion/moderna-developing-booster-shot-for-new-virus-variant-b-1-351-68384
LeBien, T. W., & Tedder, T. F. (2008). B lymphocytes: how they develop and function. Blood, 112(5), 1570–1580. https://doi.org/10.1182/blood-2008-02-078071
Teruel, N., Mailhot, O., & Najmanovich, R. J. (2020). Modelling conformational state dynamics and its role on infection for SARS-CoV-2 Spike protein variants. BioRxiv, 2020.12.16.423118. https://doi.org/10.1101/2020.12.16.423118
Wang, Z., Schmidt, F., Weisblum, Y., Muecksch, F., Barnes, C. O., Finkin, S., Schaefer-Babajew, D., Cipolla, M., Gaebler, C., Lieberman, J. A., Yang, Z., Abernathy, M. E., Huey-Tubman, K. E., Hurley, A., Turroja, M., West, K. A., Gordon, K., Millard, K. G., Ramos, V., … Nussenzweig, M. C. (2021). MRNA vaccine-elicited antibodies to SARS-CoV-2 and circulating variants. BioRxiv, 2021.01.15.426911. https://doi.org/10.1101/2021.01.15.426911
Xie, X., Zou, J., Fontes-Garfias, C. R., Xia, H., Swanson, K. A., Cutler, M., Cooper, D., Menachery, V. D., Weaver, S., Dormitzer, P. R., & Shi, P.-Y. (2021). Neutralization of N501Y mutant SARS-CoV-2 by BNT162b2 vaccine-elicited sera. BioRxiv, 2021.01.07.425740. https://doi.org/10.1101/2021.01.07.425740
Zimmer, K. (2021, January 26). A Guide to Emerging SARS-CoV-2 Variants. The Scientist Magazine®. https://www.the-scientist.com/news-opinion/a-guide-to-emerging-sars-cov-2-variants-68387
Related Posts
Genetic Cheating? Identifying CRISPR/Cas Gene Doping
Figure 1: Lance Armstrong, a well-known professional cyclist, has been...
Read MoreHow the Plague Changed Our Genes
Figure 1: An image of the Yersinia pestis bacterium that caused...
Read MoreCOVID-19 Pneumonia – A Different Illness Than Its Traditional Counterpart
Figure 1: This is an image of a child with...
Read MoreFrankie Carr


Comments are closed.